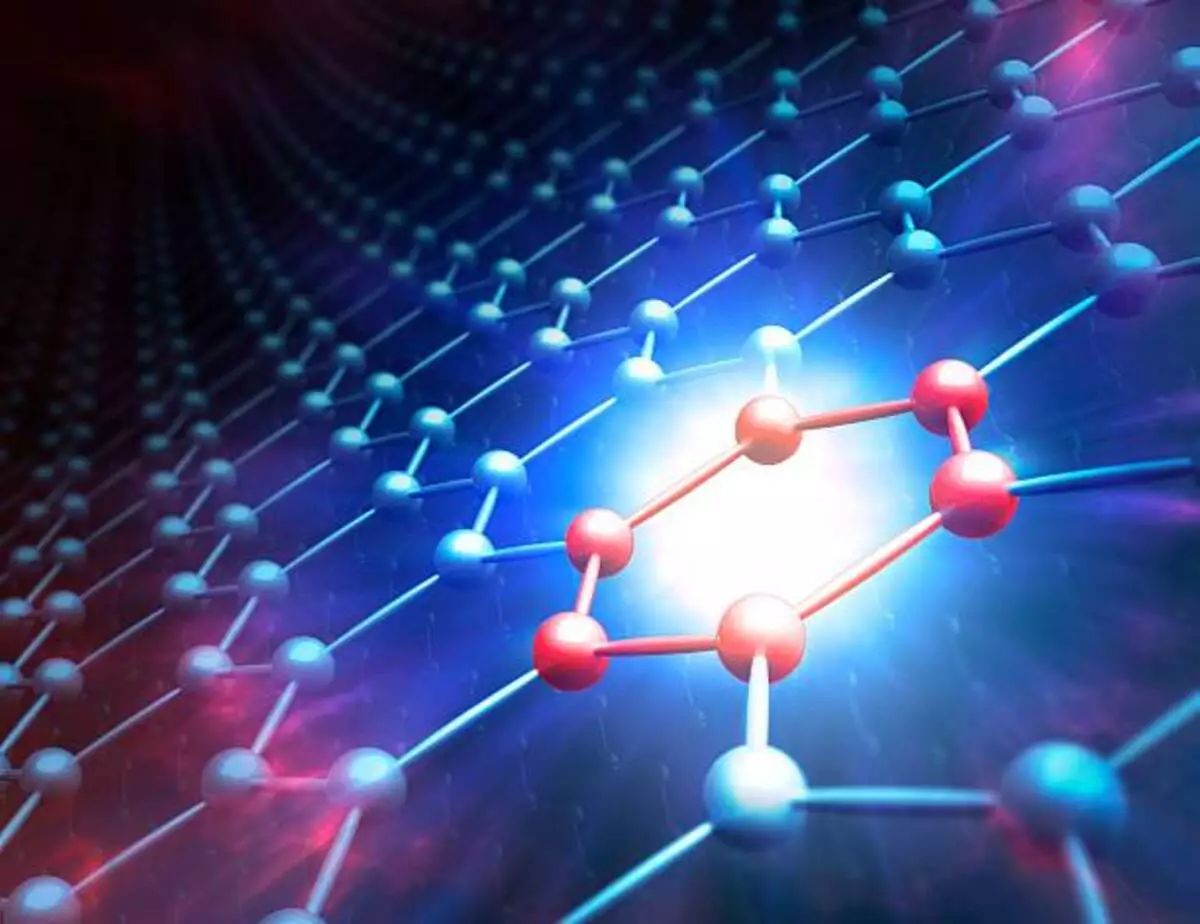
Covalent bonds are formed between atoms that share a standard pair of electrons. This bond is created by balancing the two atoms’ repulsive and attractive forces. Hydrogen is an excellent example of this type of bond. Nonmetal atoms can also form covalent bonds.
Covalent bonding is chemical bonding where atoms share a pair of electrons. The shared electrons are attracted to the nuclei of the other atom. This attraction allows the atoms to come together as one. When the attraction is strong enough, the atoms form a covalent bond. This type of bond is vital when there is room for at least two electrons in the outermost energy level.
Covalent bonds are important in organic chemistry. This bond is formed when two chemical elements share a pair of electrons. One example of this is a water molecule. A water molecule consists of two hydrogen atoms and one central oxygen atom. The oxygen atom shares one electron with each hydrogen atom.
Atoms share valence through a chemical bond, allowing them to attain a stable number of valence electrons. In a simple example, hydrogen shares an electron with another hydrogen atom, giving each atom eight electrons. These electrons come from one of the two s orbitals. The periodic table shows the electron configuration for each of these elements’ atoms.
Covalent bonds can occur between atoms of the same element and between elements with different valence. Atoms share electrons mainly to help them achieve the octet configuration and increase stability. Atoms can form different kinds of covalent bonds by sharing different numbers of electrons. For example, one type of covalent bond is formed when two atoms share one electron while another atom shares two or three electrons.
Covalent bonds are chemical bonds formed when two or more atoms share electrons. For example, hydrogen shares an electron with helium. Both atoms would happily share an electron, forming a covalent bond. This bond is the most important type of chemical bond in living things.
Hydrogen bonds are essential to life on Earth. They help hold water together, give it a lattice structure, and bind DNA’s double helix together. Chemists think a lot about them in their work studying the properties of water and aqueous acid solutions. They also wonder just how close hydrogen bonds come to be covalent.
Covalent bonds are classified as nonpolar or polar based on their electronegativity differences. FFor a nonpolar covalent bond, the difference in electronegativity between the two atoms must be greater than 0.4 on the Pauling scale.
Covalent bonds are formed between two nonmetal atoms, which share electrons. A nonpolar covalent bond is characterized by an equal distribution of electrons, with little difference between the atoms’ electronegativities. The octet rule helps explain why nonmetal atoms form covalent bonds.
In this chemical bond, nonmetals share their valence electrons. Because nonmetals have high ionization energies and high electron affinities, they can easily exchange electrons with each other. The nonmetal atoms hold shared electrons between their nuclei, forming a nonmetal compound. The nonmetal molecules are known as covalent species.
Covalent bonds are the most common chemical bond between nonmetals and metals. Nonmetals can form one to three covalent bonds with one another. The difference between the two types of covalent bonds determines the element’s reactivity. A nonmetal and a metal can form a covalent bond if they have electronegativity differences of less than 2.0 Pauling units.
A covalent bond is formed when two atoms share valence electrons. These shared electrons are attracted to the nuclei of both atoms. This is known as the octet rule. The number of shared electrons determines the number of bonds that can be formed between the atoms. For example, an oxygen atom has two pairs of bonding electrons, while a hydrogen atom shares only one pair of electrons.
The number of shared electrons in a covalent molecule is significant because it can affect the bond’s strength. Generally, the number of shared electrons is greater than the number of atoms in the molecule. This is because covalent bonds are directional solid bonds.
The vibrant universe of online casino games is teeming with a plethora of options that…
In the bustling world of online casinos, OLXTOTO stands out as a popular platform for…
Dubai, with its iconic skyline, golden deserts, and architectural marvels, is a dream destination for…
Introduction Travel has always been a gateway to personal growth and unforgettable memories. For many,…
Introduction Asphalt paving plays a crucial role in shaping the infrastructure of Moreno Valley. Whether…
Hello there, adventurous gamer! If you’ve been exploring the vast universe of online slots, chances…